
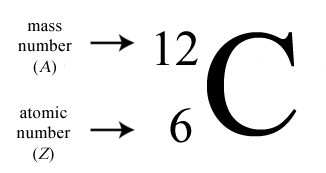
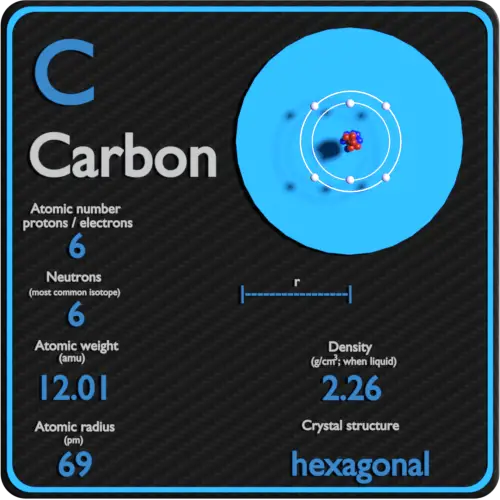
Its atomic number is 6 its atomic weight is 12.011. What element atomic number is 6 Carbon is a chemical element. Its familiar as a pure element, as diamond, graphite, and charcoal. This nonmetal is the basis for life as we know it.
The simplest organic molecules consist of carbon chemically bonded to hydrogen. Here are 10 interesting carbon facts for you: Carbon is the basis for organic chemistry, as it occurs in all living organisms. Carbon is the element with atomic number 6 and element symbol C. The new atom is an atom of potassium instead. Carbon the element that is atomic number 6 on the periodic table. One of the most important elements for all living things is carbon. If you add a proton to a carbon atom, you will not have carbon anymore - it will be nitrogen! If you take a proton away from a calcium atom, it will not be calcium anymore. This is very important to know, because it is the number of protons in the nucleus that determines what element you are working with. Every atom of lithium contains three protons in its nucleus. Every atom of helium has two protons in its nucleus. The upper number represents the - mass number & the lower number the - atomic number. For example, every atom of hydrogen has one proton in its nucleus - always. See how the atomic number increases by one for each box as you move across the table in a period? This means that each element has its own special number of protons in each atom. For example, 100g of Fe-7wtC contains 7g of Carbon and 93g of Iron. It represents the number of protons found in the nucleus of one atom of that particular element. Carbon-14, an isotope with a half-life of 5715 years, has been widely used to date such materials as wood, archaeological specimens, etc. This is the atomic number of the element. In 1961 the International Union of Pure and Applied Chemistry adopted the isotope carbon-12 as the basis for atomic weights. First, there is an integer (whole number) in some part of the box. Number the chain consecutively, starting at the end nearest a substituent group.
#CARBON ATOMIC NUMBER SERIES#
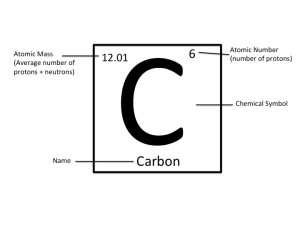
Notice that no matter what form of the table you are using, there are always three very specific items that appear for each element. Each successive member of the series has one more Carbon atom than the. Look very carefully at the boxes in the periodic table.


 0 kommentar(er)
0 kommentar(er)
